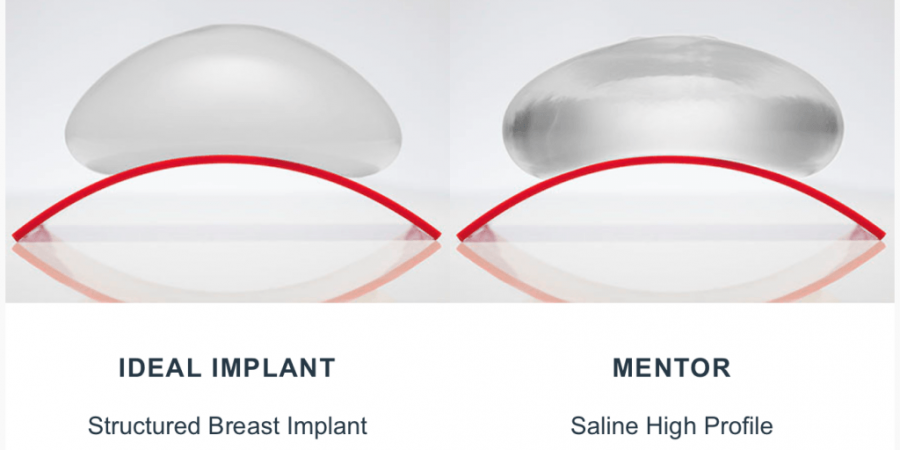
Health Canada, along with the FDA in the USA, finally approved the Ideal®Implant, for general use, a week ago. Those of us involved are very excited about this new development and being able to offer a better saline filled device to our patients.
The company has told us it will take time to ramp up production, and I will likely be able to start using the implant for my patients in about six months.
The hope is this offer a device that causes less visible rippling but still has the profoundly reassuring safety of saline fill, meaning that in the event of a leak, 10, 15, 25 years after treatment, it will be easily detected by the patient, without any special tests, and will be without doubt, a harmless leak.
More information will be coming in the next few weeks.

Dr. Gelfant’s Living Beautifully Blog
Join our mailing list and receive updates when a new blog is posted by Dr. Benjamin Gelfant.